Metabolite-protein interactions in metabolic disease
Understanding the molecular basis of metabolic diseases has become critical for the development of next-generation therapeutic strategies. My research program lies at the intersection of chemical proteomics, metabolomics, and computational strategies to uncover the intricate interplay between metabolites, proteins, diet, and gut microbiota in different metabolic disease states. By developing and leveraging innovative tools—including the Proteome Integral Solubility Alteration (PISA) assay and the multifaceted PISA-REX platform—we provide unprecedented insights into protein expression, stability, and redox regulation, which will revolutionize our understanding of metabolite interactome, key biological processes, and disease mechanisms.
Combining advanced proteomics and classical biochemical approaches, we seek to identify and validate therapeutic targets and biomarkers in metabolic diseases. In our long view of our studies, these findings are meant to be translational in the area of precision therapies and diagnostics, enhancing patient outcomes and quality of life.
Next generation microbiome-mimetic therapeutics
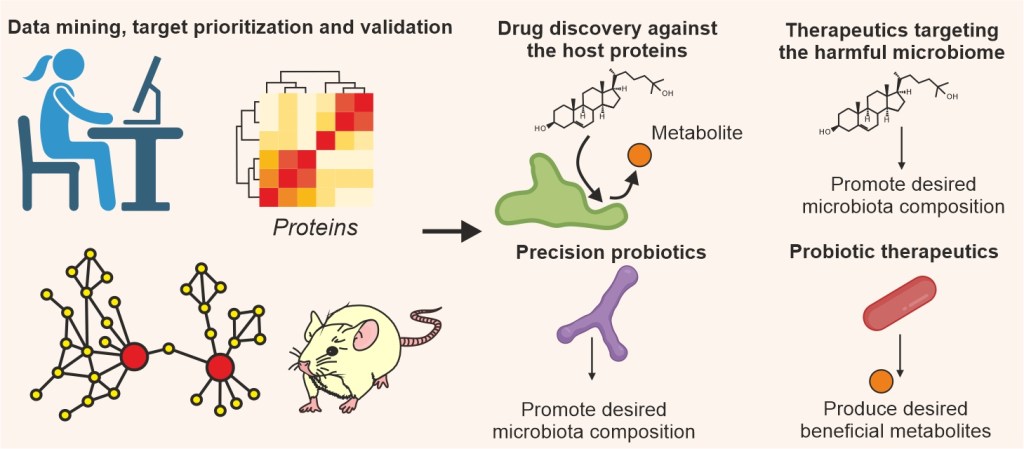
We study the complex interplay between host cells, intracellular metabolites, microbiome and microbial metabolites in the gut using a data-driven approach. Our aim is to identify metabolic vulnerabilities that can be exploited for developing novel drugs and therapeutic strategies against metabolic disorders. Our target selection approaches can be used to stratify patients and are therefore suited for precision medicine. We work at the intersection of multiple disciplines including proteomics, metabolomics, rRNA sequencing, cell biology (e.g. CRISPR-Cas9), biochemistry and biophysics (e.g. NMR, Cryo-EM, DSF and ITC) and validate our results in relevant in vitro and in vivo model systems and patient material.

We are aiming to develop novel modalities including small molecule drugs targeting host proteins as well as antibiotics and precision probiotics targeting microbiota. Target prioritization through crunching data is thus essential. We combine in-house data with those from patients and model systems to identify druggable targets based on our robust target ranking strategies (Redox Biology 2020, Advanced Science 2024). The lab is adept in employing sophisticated algorithms such as orthogonal partial least square modeling for target identification (Nature Communications 2019 and 2021, Biosensors and Bioelectronics 2023).
Development of novel proteomics tools
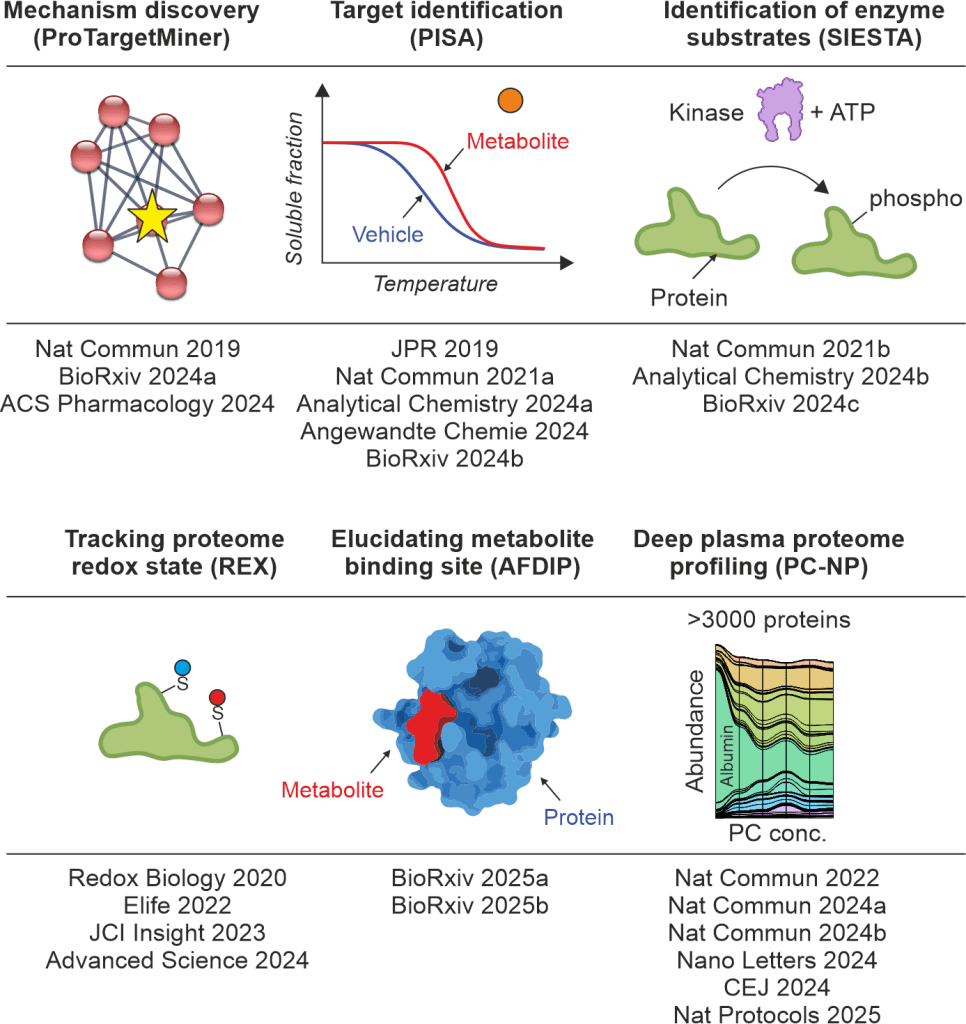
The propelling engine for our studies is chemical proteomics that can be used for studying the interaction of molecular entities with proteins. We have developed multiple techniques and methodologies including Proteome Integral Solubility Alteration (PISA) assay and its automated version OPTI-PISA , ProTargetMiner, SIESTA and GAPPIS that can be employed for target deconvolution, showcased in multiple publications for target identification and deciphering disease mechanisms.
We are also interested in applying these techniques to decipher antibiotic mode of action, and to combat antibiotic resistance.
Our latest development is the multifaceted tool PISA-REX which can profile a given proteome at the three dimensions of expression, stability/solubility and redox state (JCI Insight, 2023, Advanced Science 2024). PISA-REX is an industry standard tool for target deconvolution at the cell and tissue levels.
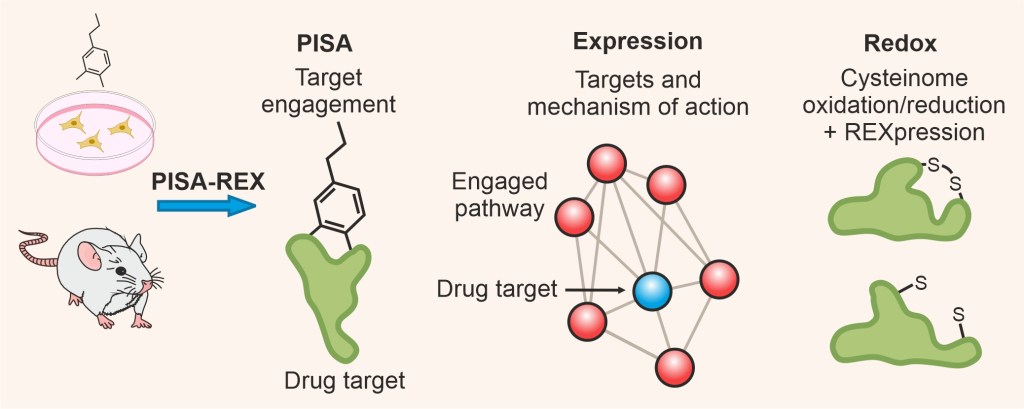
We continue working on method development in mass spectrometry-based proteomics, to enable target identification and validation. Several tools will be joining the chemical proteomics arsenal soon.
Diagnostics and biomarker discovery
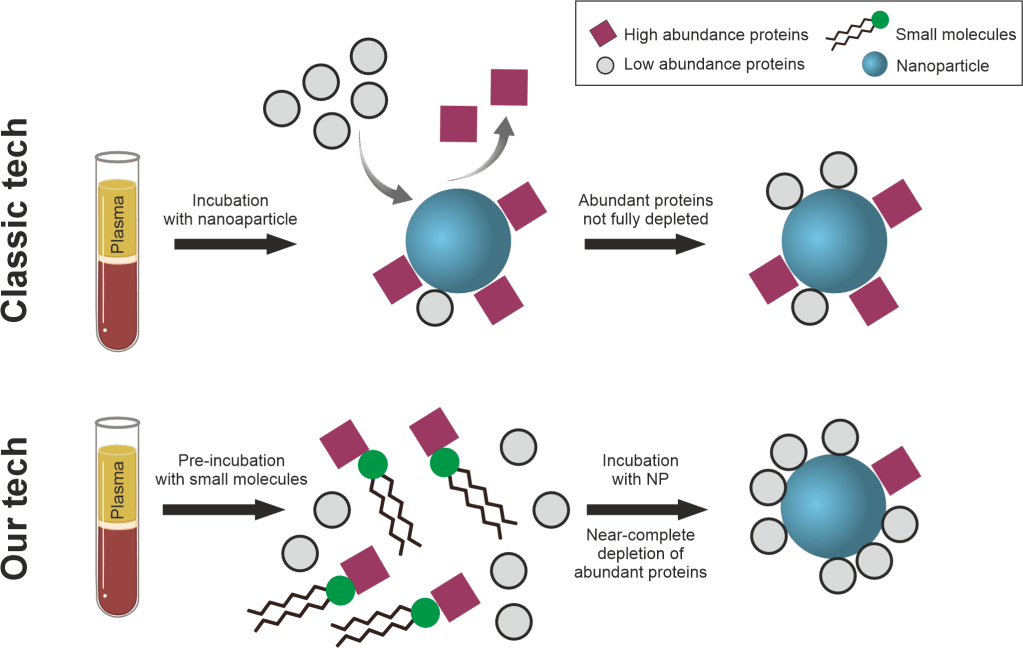
We have been working toward standardization of LC-MS methodology, data extraction and processing to enable biomarker discovery (Nature Communications 2022 and 2024a). We are also interested in multi-omics studies for discovering new biomarkers (Biosensors and Bioelectronics 2023). A current project is focused on significantly enhancing the plasma proteome coverage leveraging the interaction of metabolites such as phosphatidylcholine with plasma proteins in the presence of nanoparticles (Nature Communications 2024b). This approach can quantify up to 4500 proteins in plasma, devoid of contamination by extracellular vesicle proteins.
Advancing metaproteomics
Metaproteomics faces several challenges including the complexity of the intestinal microbiome, high dynamic range of proteins in such complex communities comprising hundreds or thousands of species and the unavailability of complete databases due to lack of genomic sequences. We will contribute to the advancement of technologies, software and algorithms to enable robust metaproteomics analysis.
“Our ultimate aim is to prevent or treat disease and enhance patients’ quality of life.”
We currently collaborate with many labs in Sweden and also have several international collaborators covering areas such as proteomics, bioinformatics, metabolism, structural biology, enzyme kinetics and tracing of isotopically-labeled metabolites: